Simplifying diagnosis: A comprehensive exploration of thyroid function test interpretation
By Dr Phoebe Stanford
Published January 2024
Thyroid function tests commonly refer to measurements of thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3). These are measured by immunoassays, which involve antibodies targeting a particular part of the hormone. These assays are not standardised, and differences in assay design and in the nature of the antibodies used may cause slight differences in results between different methods. There may also be method-specific interferences in some cases.
Who to test?
While there is insufficient evidence to support routine population screening, targeted testing in high-risk groups is recommended (see Table 1) (Garber J, 2012). As the symptoms of thyroid disease are often non-specific, thyroid function testing may have utility in the investigation of a number of clinical presentations and some biochemical changes (see Table 2).
| Table 1. High-risk groups that warrant thyroid function testing in asymptomatic individuals: |
|---|
| - Autoimmune diseases (type 1 diabetes, Addison’s disease, pernicious anaemia) - Family history of thyroid disease - Down syndrome - Turner syndrome - History of neck radiation - Iodine deficiency or high iodine load - Medications that may cause thyroid dysfunction: Lithium, Amiodarone, Immune checkpoint inhibitors |
(Royal College of Pathologists of Australasia, 2017)
| Table 2. Features of thyroid dysfunction | |
|---|---|
| HYPOTHYROIDISM | HYPERTHYROIDISM |
| Biochemical abnormalities | Biochemical abnormalities |
| - Hypercholesterolaemia - Hyperprolactinaemia (primary hypothyroidism) - Hyponatraemia - Mild anaemia | - Low cholesterol - Abnormal liver enzymes - Increased ALP of bone origin - Hypercalcaemia |
| Clinical features | Clinical features |
| General effects - Fatigue - Weight gain - Cold intolerance - Hair loss Skin and connective tissue - Dry skin, brittle nails - Non-pitting oedema Gastrointestinal - Constipation Cardiovascular - Bradycardia - Pericardial effusion Musculoskeletal - Myopathy - Arthralgia Neurological/psychiatric - Depression - Impaired memory/cognitive decline - Neuropathy (Carpal tunnel syndrome) Respiratory - Sleep apnoea - Pleural effusion Reproductive system - Impaired fertility - Menorrhagia - Oligo-amenorrhoea | General effects - Fatigue - Weight loss - Heat intolerance - Sweating, tremor Ocular - Lid retraction - Opthalmopathy (Graves’ disease) Gastrointestinal - Increased stool frequency Cardiovascular - Tachycardia - Atrial fibrillation - Heart failure Musculoskeletal - Proximal myopathy - Osteoporosis Neurological/psychiatric - Anxiety - Depression - Insomnia Reproductive system - Oligo-amenorrhoea |
Thyroid function testing should not be performed during acute illness unless there is a high index of suspicion, as acute illness alone can affect thyroid function test results, making these difficult to interpret.
What to test?
In most circumstances, screening with TSH alone is sufficient:
- with FT4 to be tested if TSH is elevated,
- and FT4 and FT3 if TSH is low.
For this cascade testing to be performed automatically under current MBS requirements, TFT should be requested rather than TSH.
The rationale for this approach is that the relationship between FT4 and TSH is not linear, with a greater change in TSH for a given change in FT4, making the TSH measurement a sensitive marker for thyroid dysfunction.
There are certain scenarios where this approach is not valid, including when secondary (central) hypothyroidism due to hypothalamic/pituitary disease is suspected. Secondary hypothyroidism will result in a low or inappropriately normal TSH, along with a low FT4. If this is suspected, initial testing with TSH and FT4 is recommended.
Thyroid antibodies
Thyroid antibodies, including Thyroid peroxidase (TPO) and Thyroglobulin (Tg) antibodies, are present in most cases of autoimmune lymphocytic “Hashimoto’s” thyroiditis, the most common cause of hypothyroidism in iodine-sufficient areas, including Australia.
TPO antibodies are more sensitive and specific than Tg antibodies for diagnosing thyroid dysfunction in autoimmune thyroiditis (O’Leary PC, 2006; Ralli M, 2020). Therefore, measuring TPO antibodies is generally sufficient for investigating hypothyroidism. TPO antibodies do not need to be repeated once positive, as there is no value in serial monitoring of TPO antibody levels.
Antithyrogloblin antibodies are recommended when measuring thyroglobulin is required for the follow-up of patients with thyroid cancer because their presence can falsely lower the thyroglobulin result due to analytical interference.
TSH receptor antibodies (TRAb) or TSH receptor stimulating immunoglobulin (TSI) are useful in investigating hyperthyroidism, whether it’s subclinical or overt. A positive TRAb or TSI is consistent with Grave’s disease. The presence of TRAb or TSI is also crucial during pregnancy, as they may cross the placenta and affect the foetal thyroid.
Understanding the impact of biotin interference in immunoassays for thyroid function testing
Biotin may interfere in immunoassays that use biotin-streptavidin interaction as part of the assay
methodology. In such assays, high doses of biotin may result in falsely high FT4 and FT3 and/or
falsely low TSH, mimicking hyperthyroidism. There are numerous cases of patients being inappropriately
investigated and treated for hyperthyroidism due to this (Kummer A, 2016; Elston MS, 2016). As such,
it is important to consider the clinical presentation when interpreting test results and question the
results if they are out of keeping with the clinical picture.
Biotin interference is not a
concern with standard multivitamin doses. However, doses of 5-10 mg or more, which may be present in
over-the-counter hair and skin supplements, have been shown to affect some assays (Haslam S, 2019).
Since the effect of biotin is assay-specific, if a patient is taking biotin, it is best to check with
the laboratory as to whether the method is affected. To avoid interference, stopping biotin for 3 days
before testing is generally sufficient, although a longer period may be required in the case of very
high doses.
Interpretation of thyroid function tests
Reference intervals are dependent on method and age
As TSH and thyroid hormone assays are not standardised, there will be slight differences in the reference intervals between methods, particularly for FT4 and FT3. Variations in reference intervals between laboratories using the same methods may also occur, depending on the characteristics of the population used to define those reference intervals.
Several studies have found that the upper limit of the reference interval for TSH is increased in the elderly. Large US-based population studies have identified a shift in the entire TSH distribution curve towards higher TSH for ages 70-89 compared to the 20-39 age group, with the TSH upper reference limit increasing to 7.5 mIU/L in those over 80 years (Surks M, 2007).
Patterns of thyroid function tests
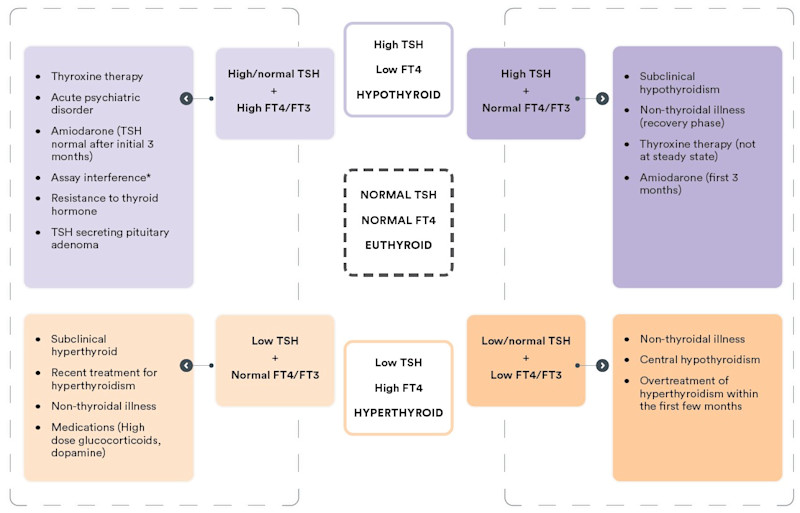
Figure 1 – Interpreting thyroid function test results at-a-glance
*Assay interference may result in artefactually low or high TSH, FT4, and/or FT3, which may result in any of the above patterns. If results do not fit with the clinical picture, and assay interference is suspected, contact the laboratory.
High TSH with low or normal FT4 and FT3:
The biochemical picture of an elevated
TSH with a low FT4 is consistent with overt primary hypothyroidism. A more common finding is an
elevated TSH with FT4 within the normal range. This may be due to subclinical hypothyroidism; however,
this may also be a transient effect reflecting recovery from non-thyroidal illness, and may also be
seen during the first few months of treatment with amiodarone. Moreover, a mildly elevated TSH (< 7
mIU/L) in patients >65 years may be considered a normal manifestation of ageing (Garber J,
2012).
A significant proportion of individuals with a mildly increased TSH (< 10mIU/L) with normal FT4 will revert to normal without treatment (Meyerovitch J, 2007). Therefore, such results should be confirmed with repeat testing of TSH together with FT4 and TPO antibodies after 6-8 weeks.
Low TSH with elevated FT4 and/or FT3:
A suppressed TSH (generally undetectable)
with elevated FT4 and/or FT3 is consistent with a diagnosis of thyrotoxicosis. This may be due to
increased production of thyroid hormones (hyperthyroidism), commonly due to Grave’s disease, toxic
multinodular goiter, or toxic adenoma, or due to release of pre-formed thyroid hormone due to
destructive thyroiditis (subacute, silent, or lymphocytic). It is important to establish the cause, as
the treatment approach differs. Thyrotoxicosis may also be factitious, due to excess use of exogenous
thyroid hormones, which may be deliberate or unintentional (potentially included in natural therapies
or weight loss supplements bought overseas), or due to excess iodine intake.
Testing of TSH receptor antibodies (TRAb or TSI) is recommended as a first-line diagnostic test. A positive TRAB or TSI is consistent with Grave’s disease. If TRAb/TSI is negative, a nuclear medicine study of the thyroid may be considered.
Low TSH with normal FT4 and FT3:
A low TSH with normal FT4/FT3 may occur
transiently with non-thyroidal illness or represent mild, subclinical hyperthyroidism, so repeat
testing should be performed after 6-8 weeks for confirmation.
Subclinical hyperthyroidism may be caused by the same pathology as overt hyperthyroidism. The approach to management depends on the risk for adverse outcomes and the degree of TSH suppression. Asymptomatic patients under age 65 with no history of cardiac disease or osteoporosis and with mildly suppressed TSH (0.1-0.4 mIU/L) may be monitored every 6-12 months. Treatment may be beneficial for older patients or those with a history of, or risk factors for heart disease or osteoporosis and is recommended if TSH is persistently < 0.1 mIU/L (Ross D, 2016). Specialist endocrine referral is recommended where treatment is being considered.
Low or normal TSH with low FT4/FT3:
A low TSH with low FT4/FT3 may be due to
severe nonthyroidal illness. If the patient has no obvious systemic illness, central hypothyroidism
due to hypothalamic or pituitary disease should be considered. This is important not to miss, as there
may be potentially life-threatening concomitant adrenal insufficiency which may be precipitated by
treatment for hypothyroidism before commencing glucocorticoid replacement.
If central hypothyroidism is suspected, pituitary hormone testing (morning cortisol, LH, FSH, sex steroids, IGF-1 and prolactin) and endocrine referral is recommended.
Unexpected results - High TSH with elevated FT4 and/or FT3:
An elevated TSH
with a high FT4 (or FT3) may occur transiently due to non-thyroidal illness, amiodarone therapy, acute
psychiatric illness, or during treatment for hypothyroidism where a steady state has not yet been
achieved, possibly due to intermittent adherence to thyroxine therapy. Depending on the clinical
presentation, a repeat test in 6-8 weeks may be a reasonable approach.
This pattern of thyroid function may also be due to an antibody interference in the test method. If this is suspected, discussion with the laboratory is recommended.
Rarely, an elevated TSH with high FT4 may be due to Resistance to thyroid hormone (RTH), a genetic condition inherited in an autosomal dominant fashion, or a TSH-producing pituitary tumour (TSHoma). If assay interference has been excluded, and this is suspected, specialist referral is recommended.
If you enjoyed this article, subscribe to our electronic Pathology Focus newsletter.
Subscribe Today!
References
Elston MS, S. S. (2016). Factitious Graves’ Disease Due to Biotin Immunoassay Interference- A Case and Review of the Literature. 101: 3251-3255.
Garber J, C. R. (2012). Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice, 18(6), 988-1028.
Haslam S, O. J. (2019). A comparison of biotin interference in routine immunoassays on the Roche Cobas 8000, Beckman Coulter DXi and Siemens Advia Centaur XPT immunoassay platforms. 57(11:e287-e290).
Kummer A, H. D. (2016). Biotin Treatment Mimicking Graves’ Disease. 375(7: 704-706).
Meyerovitch J, R.-P. P. (2007). Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med., 167(14), 1533-1538.
O’Leary PC, F. P. (2006). Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. 64: 97-104.
Ralli M, A. D. (2020). Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic T protocols, therapeutic strategies, and potential malignant transformation. Autoimmunity Reviews, 19(10), 1568-9972.
Ross D, B. H. (2016). 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid, 26(10), 1343-1421.
Royal College of Pathologists of Australasia. (2017, July). Position Statement: Thyroid function testing for adult diagnosis and monitoring. Retrieved June 2023, from https://www.rcpa.edu.au/getattachment/8d6639b7-af88-403c-a9abc5fe729757c1/Thyroid-Function-Testing-for-Adult-Diagnosis-and-M.aspx
Surks M, H. J. (2007). Age-Specific Distribution of Serum Thyrotropin and Antithyroid Antibodies in the U.S. Population: Implications for the Prevalence of Subclinical Hypothyroidism. The Journal of Clinical Endocrinology & Metabolism, 92(12), 4575-4582.