The National Cervical Screening Program aims to prevent cervical cancer through regular testing. Women and people with a cervix from 25 to 74 years of age are encouraged to have a Cervical Screening Test (CST) every five years to check for the Human Papillomavirus (HPV). The CST checks for HPV, a common sexually transmitted infection that can lead to cell changes in the cervix. Occasionally, the presence of abnormal cells can develop into cervical cancer. We accept two patient options for HPV testing:

- Clinician-Collected Samples (including for Liquid Based Cytology/LBC)
- Self-Collected Samples (or Dry Swabs)
-
What age is recommended for the first HPV screening?
-
What is the cervical screening clinical management pathway?
-
What is the difference between Liquid Based Cytology (LBC) and Dry Swab?
-
How do I order a CST at Clinical Labs?
-
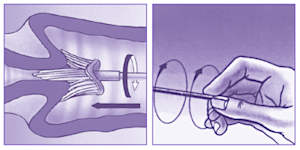
What are the LBC/ThinPrep® collection instructions at Clinical Labs?
-
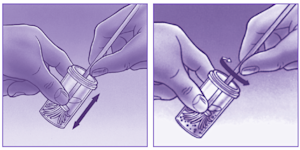
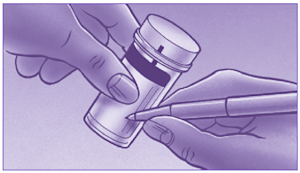
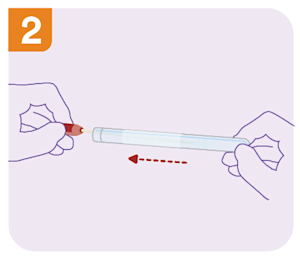
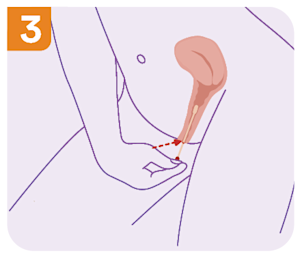
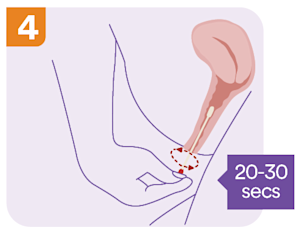
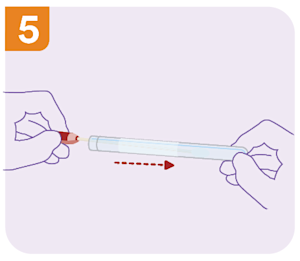
What are the self-collect instructions at Clinical Labs?
-
Patient with cervical cancer symptoms
-
How to Order HPV Testing
Who to testWomen and people with a cervix from 25 to 74 years of age should have a Cervical Screening Test every five years
Symptomatic patients, e.g. abnormal bleeding, genital warts
Asymptomatic patients with previous positive screening test
Doctor recommendation based on patient context
When to TestRoutine five-yearly screening
Doctor recommendation based on patient symptoms, context and/or age (see section How do I order a CST at Clinical Labs above)
Request Form InstructionsUse the Australian Clinical Labs General Pathology Request Form
Complete by referring to section How do I order a CST at Clinical Labs above
Record any Clinical Notes and tick relevant boxes under Cervical Cytology
Specimen DetailsRefer to section How do I order a CST at Clinical Labs for sample and test type
The relevant sections above also outline the processes for sample collection
Test costRoutine five-yearly screening – Only 1 MBS item is claimable in a 57-month period for those aged over 24yrs & 9mths
Only 1 claimable between 20 and 24 years of age
Follow-up test claimable after previous positive screening test (12-month repeat)
Only claimable within 21 months following the detection of oncogenic HPV (any type) on a self-collected screening test for those aged over 24yrs & 9mths
See section How do I order a CST at Clinical Labs for more details
Additional tests to considerThe first Cervical Screening Test at 25 years of age is an ideal opportunity to screen for common STIs, as chlamydia and gonorrhoea testing can be performed on the same ThinPrep® vial used to collect the Cervical Screening sample. No extra specimen collection is required.